Introduction: Multiple myeloma (MM) is a plasma cell dyscrasia characterized by neoplastic proliferation of plasma cells. Despite advances in treatment, therapeutic uncertainties persist. The addition of a fourth drug with the standard regimen gives a favorable prognosis and efficacy profile in MM. This systematic review highlights four-drug regimens' efficacy and safety for the treatment of newly diagnosed (ND) and relapsed refractory (RR) MM.
Methodology:
We performed a comprehensive literature search using four major databases (Pubmed, Cochrane Library, Embase, and ClinicalTrials.gov). Our search strategy included MeSH terms and keywords for multiple myeloma and antineoplastic agents from the date of inception to June 2020. The initial search revealed 6104 articles. After excluding review articles, duplicates, and non-relevant articles, we included 10 phase II/III clinical trials reporting the efficacy of four-drug regimens for treatment of MM.
Results:
Out of 2464 enrolled patients, 2321 patients had NDMM, and 143 patients had RRMM, 1791 patients were in phase III trials, and 673 patients were in phase II trials.
Phase III trials:
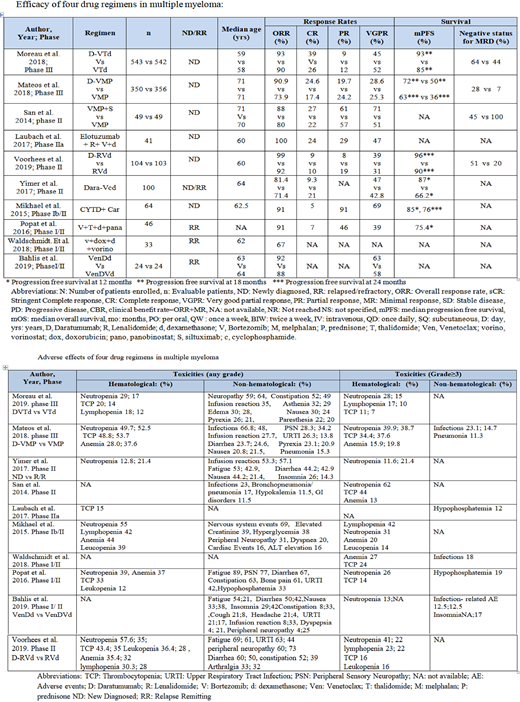
Moreau et al. (2019) studied the efficacy of daratumumab (D), bortezomib (V), thalidomide (T) and dexamethasone (d) vs VTd in NDMM patients (pts) (n=1085) in CASSIOPEIA trial. DVTd group showed better progression free survival (PFS) 93% vs 85% at 18 months (mo) (hazard ratio (HR) 0.42 [0.34-0.51]; p<0.0001), and an overall response rate (ORR) of 92.6% vs 89.9% compared to VTd group. Minimal residual disease (MRD) negative status in bone marrow was 64% in DVTd vs 44% in VTd group (P <0.0001). Mateos et al. (2018) reported the role of DV + melphalan (M) + prednisone (P) vs VMP in NDMM pts (n=706) (ALCYONE trial). DVMP group showed better PFS: 63% vs 36% at 24 mo (HR: 0.47, 95% CI 0.33-0.67, p<0.0001), ORR: 90.9% vs 73.9%, and MRD negative status of 28% vs 7% (p<0.0001) in DVMP vs VMP group. Vorhees et al. reported DVd + lenolidamide (R) vs RVd in GRIFFIN study in transplant eligible NDMM patients (n= 207). It showed better PFS: 95.8% (95% CI, 89.2- 98.4) vs 89.8% (95% CI, 77.1-95.7) at 24 mo, an ORR of 99% [Odd's ratio (OR) in DVRd group vs VRd group (91.8%) (p=0.0160) and MRD negative status of (51% vs 20.4%) (p<0.0001) in DVRd vs VRd group.
Phase II trials:
Yimer et al. (2017) studied the role of DVd + cyclophosphamide (C) in pts with NDMM (n=86) and RRMM (n=14) with ORR of 81.4% (95% CI, 71.6-89.0%) and 71.4% (95% CI, 41.9-91.6%) respectively for NDMM and RRMM. The 12-month PFS rate was 87.0% (95% CI, 57.1-96.6%), and 12-month OS rate was 98.8% (95% CI, 92.0-99.8%) for NDMM pts. Median PFS was 13.3 (95% CI, 6.8-13.3) mo and 12-month PFS rate was 66.2% (95% CI, 32.4-86.0%) and OS of 54.5% (95% CI, 8.6-86.1%) in RRMM. respectively. San et al. (2014) studied siltuximab (S) +VMP vs. VMP in NDMM pts (n=98) with ORR of 88% (95% CI 75%, 95%) in SVMP and 80% (95% CI 66%, 90%) in VMP and PFS of 88% in both arms; though median survival and overall survival were not reached in either arm despite a median follow-up of 23.3 months & 21.9 mo respectively. Laubach et al. (2017) studied the role of elotuzumab (E) + Lenalidomide (R) + VP in NDMM pts (n=41 with ORR of 100%). Mikhael et al. (2015) studied the role of Carfilzomib (K) + CTd in NDMM pts (n=64) with ORR of 91% and median PFS of 85% [95% CI (71-93%)] and 76% [95% CI (59-87%)] at 12 mo and 24 mo and OS of 96% [95% CI (86-99%)] in NDMM pts. Bahlis et al. (2019) studied the role of venetoclax (VEN) + DVd vs. VEN-Dd (n=48) with RRMM pts with ORR of 92% and 88% in Ven-Dd and Ven-DVd group. Popat et al. (2016) studied the role of Panobinostat + VTd in RRMM pts (n=56) with of ORR of 91% (80% CI 83.4-96.2) and PFS of 75.4% after a median follow-up of 15 mo. Waldschmidt et al. (2018) studied the role of vorinostat + doxorubicin + Vd in RRMM pts (n=38) with PFS of 9.6 mo, and an ORR of 67%. With a median follow-up of 30.8 mo, OS was 33.8 mo with no safety concerns. Toxicities were hematological (cytopenias) and non-hematological (fatigue, infections) and generally mild (Table).
Conclusion:Four drug combinations, primarily daratumumab based regimens, are very effective and well-tolerated. The achievement of higher MRD negative status implies a more profound response. Long term follow-up is needed to see the effect on long-term survival.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal